Introduction
Heart failure has been the leading cause of death for several decades. When medication is no longer effective in maintaining sufficient blood flow in the body, heart transplantation becomes the only viable treatment option. However, with the decreasing availability of donor hearts, many patients with advanced cardiomyopathy are dying while awaiting transplantation. To address this issue, researchers are developing Total Artificial Hearts (TAHs) as a potential alternative for these patients. TAHs are long-term mechanical circulatory devices implanted in the chest that can replace damaged heart valves and ventricles responsible for circulating blood to various body parts, including the lungs. TAHs are designed to minimize complications such as arrhythmias, intraventricular thrombus, valvular regurgitation, and right ventricular failure [1]. Additionally, they are considered a promising solution for cases of fulminant cardiac rejection.
Total Artificial Heart Description
The total artificial heart system is basically made up of four implanted components and two external connections as mentioned in Fig.1
| A | Pump Unit |
| B | Internal controller with batteries |
| C | TET system |
| D | Compliance chamber |
| E | User interface |
| F | Power supply |
Fig. 1. The Whole Total Artificial Heart System [2]
Pump Unit: The heart is a muscular pump that consists of two independent adapted artificial ventricles. Each ventricle is constituted by a multi-layer flexible polyurethane diaphragm that works on separating the blood chamber from the air chamber.
- Implantable Controller: The purpose of the implantable controller (Fig. 1B) is:
to power and control the pump unit and compliance chamber,
to provide status information of the pump unit and compliance chamber,
to provide a battery as a backup in case of a TET system failure.
TET System: The TET system (Fig. 1C) consists of the external transmitting coil, which is the primary winding of a transcutaneous high-frequency transformer, and the implanted receiving coil, which serves as the secondary.
Compliance Chamber: (Fig. 1D) Where blood resides to improve pumping function and prevent ventricular overpressure. The compliance chamber is connected to the pump unit and its operation is controlled by the implantable controller
External User Interface and Batteries: The purpose of the external user interface (Fig. 1E) is:
to monitor the airflow and driveline pressured,
- to measure the left and right cardiac outputs [1],
- to enable adjustment of all clinically relevant parameters,
- to control the energy transmission of the TET system.
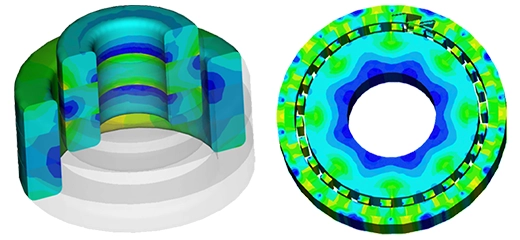
Motor Design Specifications
| Items | Symbol | Inner | Outer |
| Stator Tooth Width | w-st | 1.94 mm | 2.2 mm |
| Stator Height | hcore | 20 mm | 10.2 mm |
| Airgap | - | 0.3 mm | 0.3mm |
| Mover Tooth Pitch Ratio | w-sp/w-tp | 0.9 | 0.9 |
| Stator Slot Width Ratio | w-sl/w-st | 1.6 | 1.25 |
| PM Width Ratio | w-pm/w-st | 1 | 0.8 |
| Mover Tooth Width Ratio | w-m/w-st | 1.4 | 1.4 |
| Stator Back Iron Ratio | Hy/w-st | 2.5 | 1 |
- The DSTPM motor topology offers several advantages, including:
Eliminating the need for a transmission system prolongs the TAH's lifespan.
Low-speed operation reduces the risk of blood cell damage.
Utilizing the entire space within the mover enhances the thrust density and reduces the axial length, enabling it to fit within the limited space of the human thorax.
The double-stator topology enables the counteraction of both the inner and outer cogging forces.